Results
Between 17 January and 3 July 2020, 69 516 patients of all ages (range 0-106 years) were enrolled at 260 hospitals across England, Scotland, and Wales. Of these, 651 were patients under 19 years old with laboratory confirmed SARS-CoV-2 (651/69 516 (0.9%) of the total cohort (table 1; supplementary figures A and B). These patients were enrolled across 138 sites, of which 20 had a paediatric intensive care unit. We recruited 55 patients as hospital inpatients; they had been admitted for more than five days before symptom onset, indicating likely hospital acquired infection.
Demographics across cohort of patients under 19 years with laboratory confirmation of SARS-CoV-2. Values are numbers (percentages) unless stated otherwise
Age, sex, and ethnicity
The median age of the children was 4.6 (interquartile range 0.3-13.7) years, 35% (225/651) were under 12 months old, and 56% (367/650) were male (table 1). Ethnicity was recorded in 88% (576/651) of cases: 57% (330/576) were white, 12% (67/576) were South Asian, and 10% (56/576) were of black ethnicity. At least one comorbidity was reported in 42% (276/651) of cases.
Symptoms
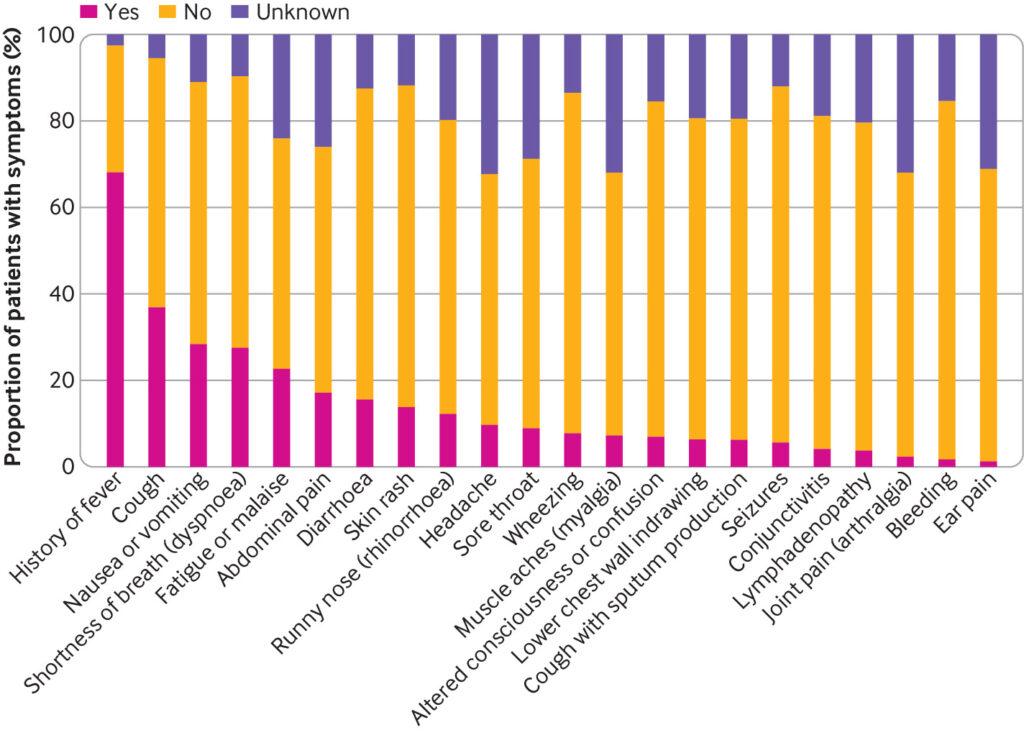
The most common presenting symptoms were fever (70%; 431/617), cough (39%; 233/599), nausea/vomiting (32%; 179/564), and shortness of breath (30%; 173/570) (fig 1). Fever and rhinorrhoea were less common with increasing age; however, nausea and vomiting, abdominal pain, headache, and sore throat showed an increasing trend with age (supplementary figure D). A heatmap and dendrogram of presenting symptoms showed three distinct clusters of clinical phenotypes (fig 2). These comprised most commonly a discrete respiratory illness (green cluster) of cough, fever, shortness of breath, runny nose, lower chest wall indrawing, and wheeze, with clustering of both upper and lower respiratory symptoms together. Next was a cluster representing a systemic mucocutaneous-enteric illness (purple cluster) of headache, myalgia, sore throat, vomiting, abdominal pain, diarrhoea, fatigue, rash, lymphadenopathy, and conjunctivitis. Finally, we observed a rarer neurological cluster of seizures and confusion (red cluster). The systemic mucocutaneous-enteric cluster includes the symptoms specified in the WHO preliminary case definition for MIS-C (“muco-cutaneous inflammation” and “acute gastrointestinal problems”), in addition to sore throat, myalgia, headache, and fatigue. The two main clusters “respiratory” and “systemic mucocutaneous-enteric” were not entirely dichotomous. Minor overlap occurred between a sub-cluster of “fever, cough, and shortness of breath” and a sub-cluster of “vomiting, abdominal pain, diarrhoea, fatigue, and rash” but very little overlap with “runny nose, wheeze, and lower chest wall indrawing.”
Proportion of patients presenting with each symptom
Heatmap with dendrogram describing clusters (coloured) of co-occurring symptoms calculated using hierarchical clustering with Jaccard distance as metric and complete linkage. Heatmap shows pairwise Jaccard indices among 20 symptoms. Jaccard index is measure of similarity that calculates ratio of number of times two symptoms appear together in data and number of times either of them appears in data. Index varies between 0 and 1, with 0 implying that two symptoms never appear together (no co-occurrence) and 1 implying that two symptoms appear only together (co-occurrence only). Dendrogram describing clusters of symptoms in heatmap was calculated using hierarchical clustering with Jaccard distance as metric and complete linkage, where Jaccard distance is calculated by subtracting Jaccard index from 1. Lower chest=lower chest wall indrawing
Comorbidities
The most common comorbidities were neurological (11%; 65/614), haematological/oncological/immunological (combined category as described in supplementary methods: 8%; 48/615), and asthma (7%; 45/615). Data on prematurity (defined as birth before completion of 37 weeks’ gestation) were as usual routinely collected only for children aged under 1 year, and 23% (46/201) were premature (table 1, supplementary table A, and supplementary figure C).
The median PEWS at presentation was 3 (interquartile range 1.0-5.0), and median blood test results at presentation were mostly within normal ranges (supplementary tables B and C). Antibiotics were given to 69% (415/601) of children, and 6% (38/591) received antiviral drugs (30 received acyclovir, 7 received remdesivir, and 3 received chloroquine/hydroxychloroquine, some in combination) (table 2).
Treatments received and outcomes. Values are numbers (percentages)
Children needing critical care
Eighteen per cent (116/632) of children were admitted to critical care (intensive care unit or high dependency unit level care); 8% (47/597) received inotropic support, 9% (57/619) received non-invasive ventilation, and 9% (58/620) received invasive mechanical ventilation (table 2, table 3, and supplementary figure E). Black ethnicity was significantly associated with admission to critical care on multivariable analysis (odds ratio 2.82, 95% confidence interval 1.41 to 5.57; P=0.003) (table 4 and supplementary table D). On multivariable analysis, both age under 1 month (odds ratio 3.21, 1.36 to 7.66; P=0.008) and age between 10 and 14 years (3.23, 1.55 to 6.99; P=0.002) were associated with admission to critical care (reference age group 15-19 years), but we found no association with sex on either univariable or multivariable analysis (table 3, table 4, and supplementary table D).
Demographics stratified by admission to critical care. Values are numbers (percentages) unless stated otherwise
Factors associated with admission to critical care unit. Values are numbers (percentages) unless stated otherwise
On univariable analysis, children with comorbidities were more likely to be admitted to critical care than those without comorbidities (odds ratio 1.73, 1.15 to 2.60; P=0.008); however, this no longer reached significance in the multivariable model (odds ratio 1.42, 0.89 to 2.28; P=0.141). Comorbidities most commonly associated with critical care admission on univariable analysis were prematurity (50% (15/30) of critical care admissions v 18% (30/165) of standard care admissions; P=0.001), respiratory comorbidities (10% (12/115) v 4% (21/491); P=0.019), cardiac comorbidities (13/115 (11%) v 25/493 (5%); P=0.018), and obesity (6% (7/115) v 2% (10/487); P=0.028) (table 3 and supplementary table E). Children receiving critical care were more likely to have been admitted to hospital more than five days before their symptoms started (indicating likely hospital acquired infection) than those receiving ward level care (16% (18/110) v 8% (36/473); P=0.009). We found no association between previous immunosuppressant use and critical care admission (table 3).
Children admitted to critical care were more likely to have presented with diarrhoea, (38% (40/104) v 13% (58/448); P<0.001), conjunctivitis (16% (16/100) v 2% (10/412); P<0.001), and altered consciousness/confusion (19% (19/99) v 6% (24/434); P<0.001) than those cared for on a standard ward (supplementary table F). They were objectively more unwell at presentation than those receiving standard ward care (median PEWS of 5 (2.0-7.0) v 2 (1.0-4.0); P<0.001) (supplementary table G). We also observed significant differences in haematological, biochemical, and radiological abnormalities between the two groups at presentation (supplementary table H). In particular, children admitted to critical care had a lower platelet count (median 192.0×109/L (interquartile range 133.0-280.5×109/L) v 296.5×109/L (229.0-383.5×109/L); P<0.001), a higher neutrophil count (7.7×109/L (4.3-12.3×109/L) v 4.6×109/L (2.3-8.2×109/L); P<0.001), and higher C reactive protein (64.5 (11.1-200.2) mg/L v 11.0 (3.0-54.5) mg/L; P<0.001) at presentation than those cared for on a standard ward. Children admitted to critical care were also more likely to have infiltrates on a chest radiograph (58% (47/81) v 32% (52/162); P<0.001).
We did a sub-analysis for critical care admission excluding any children who met the criteria for MIS-C. In this subgroup, the associations described above persisted, although obesity was no longer significantly associated with critical care admission (supplementary table I). In patients without MIS-C, prematurity and respiratory and cardiac comorbidities remained significantly associated with admission to critical care in addition to neurological (22% (17/77) v 10% (46/481); P=0.003), neurodisability (13% (9/71) v 5% (22/463); P=0.014), and gastrointestinal (9% (7/77) v 2% (11/480); P=0.007) comorbidities (supplementary table J), as is generally the case for children and young people most at risk of admission to critical care. After exclusion of children with MIS-C, conjunctivitis and diarrhoea were no longer significantly associated with critical care admission (supplementary table K); instead, shortness of breath (44% (31/71) v 28% (123/445); P=0.008) was associated with critical care admission. Total PEWS at presentation remained associated with critical care admission (supplementary table L). As expected, when cases of MIS-C were excluded, blood tests in patients admitted to critical care showed less of an inflammatory pattern compared with those cared for on a standard ward (median neutrophils 6.2×109/L (3.4-9.6×109/L) in critical care admissions versus 4.5×109/L (2.3-8.2×109/L) in standard ward admissions (P=0.059) and median C reactive protein 21.4 (5.0-51.9) mg/L v 9.0 (3.0-46.9) mg/L (P=0.069)) (supplementary table M).
Outcomes
Outcome data were available for 627 children (table 2). Six (1%) children and young people died in hospital. Three were neonates (age <28 days) with severe comorbidities/illness—very premature, complex congenital cardiac anomaly, and bacterial sepsis. Three were aged 15-18 years, two of whom had profound neurodisability with pre-existing respiratory compromise; the other was immunosuppressed by chemotherapy for malignancy and had bacterial sepsis. Two children under 5 years old, both with life limiting, complex comorbidities, were discharged with planned palliative care and cause of death was not related to covid-19. Eighty nine per cent (557/627) of children and young people were discharged alive, and 10% (62/627) continued to receive care at the date of reporting (table 2).
Patients meeting WHO preliminary case definition for MIS-C
Eleven per cent (52/456) of children met the WHO preliminary case definition for MIS-C (table 5 and supplementary figure F).9 The first patient identified developed symptoms in mid-March, when covid-19 cases were increasing nationally, followed by a small number of cases identified steadily throughout the surveillance period (fig 3). Geographically, the highest number of children with MIS-C came from areas with the largest covid-19 outbreaks—namely, the Midlands and Greater London (supplementary figure G).
Demographics at presentation and therapies administered stratified by multisystem inflammatory syndrome in children and adolescents (MIS-C) status. Values are numbers (percentage) unless stated otherwise
Dates of symptom onset for paediatric cases of SARS-CoV-2 infection and cases meeting WHO preliminary criteria for multisystem inflammatory syndrome in children and adolescents (MIS-C) in ISARIC WHO CCP-UK cohort over time
Children who met the WHO preliminary definition for MIS-C were significantly older than those who did not (median age 10.7 (8.3-14.1) v 1.6 (0.2-12.9) years; P<0.001) and were significantly more likely to be of non-white ethnicity (64% (29/45) v 42% (148/355); P=0.004) (table 5). MIS-C was associated with obesity (10% (5/51) v 2% (6/385); P=0.005) but not with any other comorbidity (supplementary table N). No children with MIS-C were admitted more than five days before symptom onset (table 5). In addition to the WHO preliminary case definition features (fever, rash, conjunctivitis, and gastrointestinal symptoms), the children with MIS-C were also more likely to present with fatigue (51% (24/47) v 28% (86/302); P=0.004), headache (34% (16/47) v 10% (26/263); P<0.001), myalgia (34% (15/44) v 8% (21/270); P<0.001), sore throat (30% (14/47) v (12% (34/284); P=0.003), and lymphadenopathy (20% (9/46) v 3% (10/318); P<0.001) than were children who did not meet MIS-C criteria (supplementary table O). Children with MIS-C also had a higher PEWS at presentation (median 5.0 (2.8-6.2) v 3.0 (1.0-5.0); P<0.001) and were more likely to have reduced consciousness (25% (13/51) v 9% (33/350); P=0.001) than those who did not meet the criteria (supplementary table P and supplementary figure H).
Children with MIS-C were five times more likely than others to be admitted to critical care (73% (38/52) v 15% (62/404); P<0.001) (table 5). They were more likely to receive intravenous corticosteroids (55% (24/44) v 6% (20/353); P<0.001), non-invasive (35% (18/52) v 8% (31/397); P<0.001) and invasive ventilation (27% (14/52) v 9% (36/397); P=0.001), and inotropic support (51% (25/49) v 5% (18/380); P=0.001) (table 5). Sixty five per cent (28/43) of patients with MIS-C received intravenous immunoglobulins, and 17% (7/42) received immunomodulatory therapy (three received anakinra, one tocilizumab, one adalimumab, one infliximab, and one unspecified) (supplementary table R). Fifty seven per cent (21/37) of MIS-C cases had one or more documented cardiac complications. Of these, 10 had impaired cardiac function on echocardiogram, nine had a pericardial effusion, three had electrocardiographic changes (heart block, junctional rhythm and T wave inversion, borderline ST elevation and changes of pericarditis), three had coronary artery dilatation, two had coronary artery aneurysm, two had myocarditis and two had valvular regurgitation. No deaths occurred in the MIS-C group.
Review of laboratory investigations found that children who met the criteria for MIS-C were more likely to have a platelet count of less than 150×109/L than those who did not (32% (16/50) v 11% (38/348); P<0.001) (supplementary table Q). Children who met the MIS-C criteria also had lower lymphocyte counts (median 0.9×109/L (0.7-1.7×109/L) v 2.2×109/L (1.3-3.8×109/L); P<0.001) but higher neutrophil counts (8.3×109/L (5.9-12.4×109/L) v 4.6×109/L (2.3-8.6×109/L); P<0.001) and higher creatinine (55.0 (35.8-82.5) μmol/L v 30.0 (20.0-51.8) μmol/L; P<0.001) than those without (supplementary table Q).
Of the 52 patients with MIS-C, 56% (28/50) were polymerase chain reaction positive for SARS-CoV-2 (acute infection), 44% (22/50) were SARS-CoV-2 antibody positive (post-acute), and two were confirmed but the method was not specified. (supplementary table R). Patients with MIS-C who were antibody positive were younger (median age 10.0 (7.7-13.2) years v 12.4 (8.9-15.3) years; P=0.057) and more likely to be of non-white ethnicity (90% (19/21) v 45% (10/22); P=0.003) than those who were polymerase chain reaction positive. Although obesity had been associated with MIS-C (table 5), when analysed by SARS-CoV-2 detection method this feature seemed to be driven by those with MIS-C who were polymerase chain reaction positive, as none of the antibody positive patients were obese (19% (5/27) v 0% (0/22); P=0.056) (supplementary table S).
On comparing presenting symptoms, we found that conjunctivitis (71% (15/21) v 16% (4/25); P<0.001) and abdominal pain (95% (20/21) v 44% (12/27); P<0.001) were more common in patients who were in the antibody positive group, whereas those in the acute phase of infection and positive by polymerase chain reaction were more likely to present with shortness of breath (52% (14/27) v 14% (3/21); P=0.014) (supplementary table T).
Although PEWS was similar between the groups, children who were polymerase chain reaction positive were more likely to have a need for oxygen at presentation (40% (10/25) v 14% (3/22); P=0.056) (supplementary table U). Blood results at presentation were similar between the two groups, with the exception of prothrombin time, which was longer in the group who were polymerase chain reaction positive (median 14.8 (13.4-15.8) sec v 11.6 (11.0-12.0) sec; P=0.013) (supplementary table V).
Treatments received also differed between the two groups, with patients who were antibody positive being more likely to receive intravenous steroids (84% (16/19) v 35% (8/23); P=0.002) and intravenous immunoglobulin (86% (19/22) v 43% (9/21); P=0.004) than those who were polymerase chain reaction positive (supplementary table R). The need for critical care or respiratory or cardiovascular support did not differ between the two groups. Although cardiac complications were seen in both groups, they were more frequent in patients with MIS-C who were antibody positive (75% (15/20) v 35% (6/17); P=0.022) (supplementary table R).
Discussion
Six hundred and fifty one children and young people aged under 19 years with laboratory confirmed SARS-CoV-2 were recruited to the ISARIC WHO CCP-UK study between 17 January and 3 July 2020, accounting for 0.9% of all patients in the whole cohort at that time. The median age of children with covid-19 was 4.6 (interquartile range 0.3–13.7) years. The cohort was predominantly male (56%) and of white ethnicity (57%), with most (58%) children having no known comorbidities. The most common presenting symptoms were fever, cough, shortness of breath, nausea, and vomiting, and a systemic mucocutaneous-enteric cluster of symptoms was seen. Eighteen per cent of children admitted to hospital needed critical care. Critical care admission was associated with age younger than 1 month, age 10-14 years, and black ethnicity. The all cause in-hospital case fatality rate for children and young people was strikingly low at 1% (6/627), compared with 27% (18 803/69 516) in the whole cohort of all ages (0-106 years) over the same time period. In this paediatric cohort, 11% of children and young people met the WHO preliminary criteria for MIS-C, which was associated with older age, non-white ethnicity, and admission to critical care. MIS-C cases were first identified in mid-March when cases of covid-19 began to rise in the UK. In addition to the clinical criteria provide by WHO,9 we found that children with MIS-C were more likely to present with fatigue, headache, myalgia, sore throat, and lymphadenopathy, as well as a lower platelet count than children with SARS-CoV-2 who did not meet the MIS-C criteria.
Children who had been admitted to hospital for more than five days before symptom onset were also more likely to be admitted to critical care. By definition, this group includes children with comorbidity, which was associated with critical care admission. SARS-CoV-2 nosocomial infections in children are not well reported, and this area warrants closer scrutiny, ideally with the use of viral sequence data.
This study identified children meeting the criteria for MIS-C in both the acute phase of infection (polymerase chain reaction positive) and post-acute or convalescent phase of infection (antibody positive) groups. Although the two groups shared many similarities, important differences included the post-acute group being more strongly associated with non-white ethnicity and muco-enteric symptom presentation (abdominal pain and conjunctivitis), whereas the acute group presented more commonly with respiratory symptoms. Cardiac complications occurred across both groups but were more common in the post-acute patients, who were also more likely to receive intravenous steroids and immunoglobulins.
Strengths and limitations of study
This study is unique in that data for patients with laboratory confirmed covid-19 were collected prospectively and throughout the admission. The ISARIC WHO CCP-UK study had previously been activated in 2016 and 2018 for cases of Middle East respiratory syndrome (MERS) and monkeypox, and so was prepared for the SARS-CoV-2 pandemic, allowing swift activation. Consequently, in addition to reporting the clinical characteristics, risk factors, and outcomes of covid-19 in children, this dataset provided a unique opportunity to objectively monitor the emergence and progression of a novel multisystem inflammatory syndrome in the UK, while minimising recall bias. The first patient meeting the criteria for MIS-C was identified on 20 March 2020, and the first published cases were reported on 6 May 2020.8 Comparison with overall covid-19 cases confirms the sporadic occurrence of MIS-C throughout the first peak of the covid-19 pandemic in the UK. In contrast to previous reports, our analysis was limited to children admitted with laboratory confirmed SARS-CoV-2, which allowed us to clearly define the picture of covid-19 in children and reduce confounding by other potential causes.
The ISARIC WHO CCP-UK database was estimated to represent two thirds of hospital admissions for covid-19 across England, Wales, and Scotland at the time of extraction. It is therefore susceptible to selection bias, particularly as tertiary centres with critical care units and specialist children’s hospitals are more likely to have dedicated research teams, potentially skewing the severity and age of the patients reported. The most common presenting symptoms in children in our study (fever, cough, and dyspnoea) reflect the original case definition for SARS-CoV-2 testing in the UK, suggesting that this paediatric cohort is likely to have been influenced by the testing criteria.
The PEWS is validated up to 16 years of age.14 As ranges of clinical observations do not vary much between 16 and 18 year olds,20 PEWS scoring was extended to all those under 19 years. To identify children and young people meeting the WHO criteria for MIS-C, data on C reactive protein and fever are needed. Decisions to measure C reactive protein and other parameters were at physicians’ discretion. Children missing either of these variables were excluded from this analysis.
A limitation of this study is the use of a case record form that was agnostic to age and so not specifically tailored for paediatric data collection, particularly regarding comorbidities. Some of this information was available in free text, but these data were incomplete. By design, we were not able to differentiate between people whose symptoms were directly attributable to SARS-CoV-2 infection and those who had been admitted for other reasons and then found to be positive for the virus. The study relied primarily on polymerase chain reaction testing as evidence of SARS-CoV-2 infection, as diagnostic serology was not available at the start of the pandemic. This could have limited early recruitment of MIS-C cases. Finally, in order to share findings from this study promptly as an urgent public health research priority, these analyses were performed on a cohort with ongoing data collection and missing data, the proportion of which will decrease with time. This may affect our estimate of the incidence of MIS-C at 11% (52/456) among children and young people with proven SARS-CoV-2 infection, which is calculated on the 70% (456/651) who had data available for fever and C reactive protein, thus allowing WHO preliminary criteria to be applied. Children without features of MIS-C or sepsis are not likely to have their clotting and inflammatory markers measured, so reducing the pool of complete data and the denominator. We do not have data for children identified as infected with SARS-CoV-2 in the community who were not admitted to hospital, and we cannot yet report on sequelae of covid-19 in children after discharge.
Comparison with other studies
Children and young people aged under 19 years accounted for 0.9% (651/69 516) of the ISARIC WHO CCP-UK cohort on 3 July 2020, which is broadly consistent with 2% reported in China and 1.7% in North America.12 Our cohort of paediatric patients admitted to hospital had a median age of 4.6 years, which was similar to an Italian cohort (3.3 years21), but younger than Chinese (6-7 years56) and North American (11 years2) cohorts; however, these other cohorts were not limited to children admitted to hospital. Although respiratory presentations were most common, 35% of children also had gastrointestinal symptoms at presentation, which is higher than the 10-22% reported in other paediatric literature.22122 Gastrointestinal symptoms have also been prominent in children presenting with infection by MERS-CoV (28%) and severe acute respiratory syndrome coronavirus (SARS-CoV) (30%).2324 We also identified a distinct systemic mucocutaneous-enteric cluster of symptoms in the acute phase of SARS-CoV-2 infection, which shows overlap with the WHO preliminary case definition for MIS-C.
Children of black ethnicity were over-represented, comprising 10% of our paediatric cohort compared with a population representation of 4.7% of all children under 18 years across England and Wales and 1% in Scotland.2526 This finding may also be influenced by the ethnic composition of the population served by the sites recruiting to this study. Black ethnicity was also associated with increased odds of admission to critical care on multivariable analysis, consistent with reports for adult populations suggesting that South Asian and black ethnicities are disproportionately severely affected by SARS-CoV-2 infection.272829 Studies of paediatric covid-19 from other countries either have been from ethnically homogenous groups or have not reported ethnicity, making comparisons difficult.
The rate of admission to critical care in our cohort was 18%, compared with 10% reported in a North American cohort of children admitted to hospital and 13% in a multicentre cohort study across 25 European countries.222 As previously noted, this rate may be elevated in our study owing to hospitals with dedicated paediatric research teams being more likely to provide paediatric critical care. The prevalence of comorbidities (54%) in children admitted to critical care in our cohort was also similar to that reported in the European multicentre study (52%).22 Obesity was associated with critical care admission in our paediatric cohort, in agreement with adult data from ISARIC WHO CCP-UK.13 In England, 20% of children are obese by 11 years of age.30 Childhood obesity, however, is also influenced by deprivation,30 which we did not analyse in our study. Age under 1 month was associated with increased odds of critical care admission, in agreement with the European cohort.22 Thirty five per cent of children in our study were under 1 year old, which may reflect a low threshold for admissions of infants by clinicians rather than severe concerns about their clinical condition. This may explain the predominance of younger children in our study. In addition, the association between age under 1 month and admission to critical care might be explained if these babies were already admitted to neonatal intensive care and undergoing regular SARS-CoV-2 screening.
Using adapted WHO criteria,9 we identified 52 patients meeting the criteria for multisystem inflammatory syndrome. Initial UK reports described children admitted to hospital with circulatory shock and a hyperinflammatory state with features similar to toxic shock or Kawasaki disease.8 Children fulfilling the case definition for MIS-C have been reported in multiple regions experiencing large outbreaks of covid-19, including England (UK),31 Paris (France),11 Bergamo (Italy),10 and New York City (USA).32 Ours is the first report, however, to identify cases and timelines by using a prospective national data collection strategy. MIS-C seems to be temporally associated with covid-19, but a causal relation remains to be established. Older age and non-white ethnicity were associated with MIS-C in our study, in agreement with a recent case series of 99 children with MIS-C from New York State (USA), where 63% were of non-white ethnicity and 69% were aged between 6 and 20 years.32 Children in our study with MIS-C were much more unwell than other children with covid-19, with 51% needing inotropic support, compared with 20% in the Italian cohort,10 47% in the French cohort,11 and 62% in the New York cohort.29
It is becoming apparent that MIS-C can present both in children with acute SARS-CoV-2 infection and in the post-acute or convalescent phase of infection. A large case series of 565 children with MIS-C across North America has been recently reported by the Centers for Disease Prevention and Control (CDC), which used latent class analysis to identify three classes of MIS-C.33 Class 1 predominantly comprises patients who were antibody positive and polymerase chain reaction negative for SARS-CoV-2, with multiple systems involved, a strong association with cardiac complications, and a greater likelihood of having received intravenous immunoglobin and corticosteroids. These class 1 patients resemble the post-acute group in our analysis. The CDC describes class 2 as children who were predominantly polymerase chain reaction positive with more respiratory involvement, and these resemble the acute group in our analysis.
We believe that the characterisation of SARS-CoV-2 illness in children into polymerase chain reaction positive versus serology positive as distinct entities is likely to be an oversimplification. Instead, our data suggest that clinical presentation varies at different time points in the course of SARS-CoV-2 infection and immune response.
Across the whole of our paediatric cohort, we identified a distinct cluster presenting with systemic mucocutaneous-enteric symptoms (rash, conjunctivitis, diarrhoea, vomiting, and abdominal pain) in addition to headache, myalgia, sore throat, fatigue, and lymphadenopathy, which overlapped closely with the WHO preliminary case definition.9
MIS-C can present in both the acute and convalescent phases of SARS-CoV-2 infection. The significant associations between MIS-C and fatigue, headache, myalgia, sore throat, and lymphadenopathy in our cohort may be useful in refining the case definition. In addition, the association of MIS-C with platelet count less than 150×109/L and low lymphocyte counts agrees with previous reports.3132 These important findings may assist in differentiating this syndrome from other illnesses, particularly Kawasaki disease in which platelet counts are typically elevated.
Conclusion and policy implications
Our data confirm less severe covid-19 in children and young people with SARS-CoV-2 infection than in adults. Admission to critical care was associated with age under 1 month, age 10-14 years, and black ethnicity. In agreement with previous reports, we found older age and non-white ethnicity to be associated with MIS-C.
We also report that patients with MIS-C who are antibody positive for SARS-CoV-2 are more likely to be of non-white ethnicity, have mucocutaneous-enteric symptoms and cardiac complications, and have received intravenous immunoglobulins and corticosteroids than those who are in the acute phase of infection (polymerase chain reaction positive).
We have identified a systemic mucocutaneous-enteric symptom cluster across the whole cohort. In addition, we have provided evidence for refining the WHO case definition for MIS-C, including an association with low platelet count, fatigue, headache, myalgia, sore throat, and lymphadenopathy.
What is already known on this topic
-
Less information on ethnicity, comorbidities, and clinical and laboratory findings is available in children with SARS-CoV-2 than in adults
-
A multisystem inflammatory syndrome in children and adolescents (MIS-C) temporally associated with SARS-CoV-2 has been widely reported
-
However, all reports to date arise from retrospective cases series that are vulnerable to ascertainment, selection, and recall bias
What this study adds
-
Severe disease was rare and death exceptionally rare in this is a large prospective cohort study of children admitted to hospital with laboratory confirmed covid-19
-
Ethnicity seems to be a factor in both critical care admission and MIS-C
-
This cohort has enabled identification of additional clinical and laboratory characteristics that should help to refine the WHO criteria for MIS-C
Acknowledgments
This work uses data provided by patients and collected by the NHS as part of their care and supports #DataSavesLives. We are extremely grateful to the 2648 frontline NHS clinical and research staff and volunteer medical students who collected this data in challenging circumstances and for the generosity of the participants and their families for their individual contributions in these difficult times. We also acknowledge the support of Jeremy J Farrar, Nahoko Shindo, Devika Dixit, Nipunie Rajapakse, Lyndsey Castle, Martha Buckley, Debbie Malden, Katherine Newell, Kwame O’Neill, Emmanuelle Denis, Claire Petersen, Scott Mullaney, Sue MacFarlane, Nicole Maziere, Emily Cass, Krishanthi S Subramaniam, Julien Martinez, Oslem Dincarslan, and Annette Lake. The study protocol is available at https://isaric4c.net/protocols; study registry https://www.isrctn.com/ISRCTN66726260.
ISARIC Coronavirus Clinical Characterisation Consortium (ISARIC4C) Investigators: Consortium lead investigator: J Kenneth Baillie; chief investigator: Malcolm G Semple. Co-lead investigator: Peter J M Openshaw. ISARIC clinical coordinator: Gail Carson. Co-investigators: Beatrice Alex, Benjamin Bach, Wendy S Barclay, Debby Bogaert, Meera Chand, Graham S Cooke, Annemarie B Docherty, Jake Dunning, Ana da Silva Filipe, Tom Fletcher, Christopher A Green, Ewen M Harrison, Julian A Hiscox, Antonia Ying Wai Ho, Peter W Horby, Samreen Ijaz, Saye Khoo, Paul Klenerman, Andrew Law, Wei Shen Lim, Alexander, J Mentzer, Laura Merson, Alison M Meynert, Mahdad Noursadeghi, Shona C Moore, Massimo Palmarini, William A Paxton, Georgios Pollakis, Nicholas Price, Andrew Rambaut, David L Robertson, Clark D Russell, Vanessa Sancho-Shimizu, Janet T Scott, Louise Sigfrid, Tom Solomon, Shiranee Sriskandan, David Stuart, Charlotte Summers, Richard S Tedder, Emma C Thomson, Ryan S Thwaites, Lance C W Turtle, Maria Zambon; project managers: Hayley Hardwick, Chloe Donohue, Jane Ewins, Wilna Oosthuyzen, Fiona Griffiths. Data analysts: Lisa Norman, Riinu Pius, Tom M Drake, Cameron J Fairfield, Stephen Knight, Kenneth A Mclean, Derek Murphy, Catherine A Shaw. Data and information system managers: Jo Dalton, Michelle Girvan, Egle Saviciute, Stephanie Roberts, Janet Harrison, Laura Marsh, Marie Connor, Sophie Halpin, Clare Jackson, Carrol Gamble. Data integration and presentation: Gary Leeming, Andrew Law, Ross Hendry. Material management: William Greenhalf, Victoria Shaw, Sarah McDonald. Outbreak laboratory volunteers: Katie A Ahmed, Jane A Armstrong, Milton Ashworth, Innocent G Asiimwe, Siddharth Bakshi, Samantha L Barlow, Laura Booth, Benjamin Brennan, Katie Bullock, Benjamin W A Catterall, Jordan J Clark, Emily A Clarke, Sarah Cole, Louise Cooper, Helen Cox, Christopher Davis, Oslem Dincarslan, Chris Dunn, Philip Dyer, Angela Elliott, Anthony Evans, Lewis W S Fisher, Terry Foster, Isabel Garcia-Dorival, Willliam Greenhalf, Philip Gunning, Catherine Hartley, Antonia Ho, Rebecca L Jensen, Christopher B Jones, Trevor R Jones, Shadia Khandaker, Katharine King, Robyn T Kiy, Chrysa Koukorava, Annette Lake, Suzannah Lant, Diane Latawiec, L Lavelle-Langham, Daniella Lefteri, Lauren Lett, Lucia A Livoti, Maria Mancini, Sarah McDonald, Laurence McEvoy, John McLauchlan, Soeren Metelmann, Nahida S Miah, Joanna Middleton, Joyce Mitchell, Shona C Moore, Ellen G Murphy, Rebekah Penrice-Randal, Jack Pilgrim, Tessa Prince, William Reynolds, P. Matthew Ridley, Debby Sales, Victoria E Shaw, Rebecca K Shears, Benjamin Small, Krishanthi S Subramaniam, Agnieska Szemiel, Aislynn Taggart, Jolanta Tanianis, Jordan Thomas, Erwan Trochu, Libby van Tonder, Eve Wilcock, J Eunice Zhang. Local principal investigators: Kayode Adeniji, Daniel Agranoff, Ken Agwuh, Dhiraj Ail, Ana Alegria, Brian Angus, Abdul Ashish, Dougal Atkinson, Shahedal Bari, Gavin Barlow, Stella Barnass, Nicholas Barrett, Christopher Bassford, David Baxter, Michael Beadsworth, Jolanta Bernatoniene, John Berridge, Nicola Best, Pieter Bothma, David Brealey, Robin Brittain-Long, Naomi Bulteel, Tom Burden, Andrew Burtenshaw, Vikki Caruth, David Chadwick, Duncan Chambler, Nigel Chee, Jenny Child, Srikanth Chukkambotla, Tom Clark, Paul Collini, Catherine Cosgrove, Jason Cupitt, Maria-Teresa Cutino-Moguel, Paul Dark, Chris Dawson, Samir Dervisevic, Phil Donnison, Sam Douthwaite, Ingrid DuRand, Ahilanadan Dushianthan, Tristan Dyer, Cariad Evans, Chi Eziefula, Christopher Fegan, Adam Finn, Duncan Fullerton, Sanjeev Garg, Sanjeev Garg, Atul Garg, Jo Godden, Arthur Goldsmith, Clive Graham, Elaine Hardy, Stuart Hartshorn, Daniel Harvey, Peter Havalda, Daniel B Hawcutt, Maria Hobrok, Luke Hodgson, Anita Holme, Anil Hormis, Michael Jacobs, Susan Jain, Paul Jennings, Agilan Kaliappan, Vidya Kasipandian, Stephen Kegg, Michael Kelsey, Jason Kendall, Caroline Kerrison, Ian Kerslake, Oliver Koch, Gouri Koduri, George Koshy, Shondipon Laha, Susan Larkin, Tamas Leiner, Patrick Lillie, James Limb, Vanessa Linnett, Jeff Little, Michael MacMahon, Emily MacNaughton, Ravish Mankregod, Huw Masson, Elijah Matovu, Katherine McCullough, Ruth McEwen, Manjula Meda, Gary Mills, Jane Minton, Mariyam Mirfenderesky, Kavya Mohandas, Quen Mok, James Moon, Elinoor Moore, Patrick Morgan, Craig Morris, Katherine Mortimore, Samuel Moses, Mbiye Mpenge, Rohinton Mulla, Michael Murphy, Megan Nagel, Thapas Nagarajan, Mark Nelson, Igor Otahal, Mark Pais, Selva Panchatsharam, Hassan Paraiso, Brij Patel, Justin Pepperell, Mark Peters, Mandeep Phull, Stefania Pintus, Jagtur Singh Pooni, Frank Post, David Price, Rachel Prout, Nikolas Rae, Henrik Reschreiter, Tim Reynolds, Neil Richardson, Mark Roberts, Devender Roberts, Alistair Rose, Guy Rousseau, Brendan Ryan, Taranprit Saluja, Aarti Shah, Prad Shanmuga, Anil Sharma, Anna Shawcross, Jeremy Sizer, Richard Smith, Catherine Snelson, Nick Spittle, Nikki Staines, Tom Stambach, Richard Stewart, Pradeep Subudhi, Tamas Szakmany, Kate Tatham, Jo Thomas, Chris Thompson, Robert Thompson, Ascanio Tridente, Darell Tupper-Carey, Mary Twagira, Andrew Ustianowski, Nick Vallotton, Lisa Vincent-Smith, Shico Visuvanathan, Alan Vuylsteke, Sam Waddy, Rachel Wake, Andrew Walden, Ingeborg Welters, Tony Whitehouse, Paul Whittaker, Ashley Whittington, Meme Wijesinghe, Martin Williams, Lawrence Wilson, Sarah Wilson, Stephen Winchester, Martin Wiselka, Adam Wolverson, Daniel G Wooton, Andrew Workman, Bryan Yates, Peter Young.