Summary
Background
To date, few data on paediatric COVID-19 have been published, and most reports originate from China. This study aimed to capture key data on children and adolescents with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection across Europe to inform physicians and health-care service planning during the ongoing pandemic.
Methods
This multicentre cohort study involved 82 participating health-care institutions across 25 European countries, using a well established research network—the Paediatric Tuberculosis Network European Trials Group (ptbnet)—that mainly comprises paediatric infectious diseases specialists and paediatric pulmonologists. We included all individuals aged 18 years or younger with confirmed SARS-CoV-2 infection, detected at any anatomical site by RT-PCR, between April 1 and April 24, 2020, during the initial peak of the European COVID-19 pandemic. We explored factors associated with need for intensive care unit (ICU) admission and initiation of drug treatment for COVID-19 using univariable analysis, and applied multivariable logistic regression with backwards stepwise analysis to further explore those factors significantly associated with ICU admission.
Findings
582 individuals with PCR-confirmed SARS-CoV-2 infection were included, with a median age of 5·0 years (IQR 0·5–12·0) and a sex ratio of 1·15 males per female. 145 (25%) had pre-existing medical conditions. 363 (62%) individuals were admitted to hospital. 48 (8%) individuals required ICU admission, 25 (4%) mechanical ventilation (median duration 7 days, IQR 2–11, range 1–34), 19 (3%) inotropic support, and one (<1%) extracorporeal membrane oxygenation. Significant risk factors for requiring ICU admission in multivariable analyses were being younger than 1 month (odds ratio 5·06, 95% CI 1·72–14·87; p=0·0035), male sex (2·12, 1·06–4·21; p=0·033), pre-existing medical conditions (3·27, 1·67–6·42; p=0·0015), and presence of lower respiratory tract infection signs or symptoms at presentation (10·46, 5·16–21·23; p<0·0001). The most frequently used drug with antiviral activity was hydroxychloroquine (40 [7%] patients), followed by remdesivir (17 [3%] patients), lopinavir–ritonavir (six [1%] patients), and oseltamivir (three [1%] patients). Immunomodulatory medication used included corticosteroids (22 [4%] patients), intravenous immunoglobulin (seven [1%] patients), tocilizumab (four [1%] patients), anakinra (three [1%] patients), and siltuximab (one [<1%] patient). Four children died (case-fatality rate 0·69%, 95% CI 0·20–1·82); at study end, the remaining 578 were alive and only 25 (4%) were still symptomatic or requiring respiratory support.
Interpretation
COVID-19 is generally a mild disease in children, including infants. However, a small proportion develop severe disease requiring ICU admission and prolonged ventilation, although fatal outcome is overall rare. The data also reflect the current uncertainties regarding specific treatment options, highlighting that additional data on antiviral and immunomodulatory drugs are urgently needed.
Funding
ptbnet is supported by Deutsche Gesellschaft für Internationale Zusammenarbeit.
Introduction
WHO
Novel coronavirus (2019-nCoV) situation report 5.
Coronaviridae Study Group of the International Committee on Taxonomy of Viruses
The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2.
Currently, there are no antiviral treatment options with proven efficacy, but several randomised controlled trials are investigating agents such as hydroxychloroquine, lopinavir–ritonavir, favipiravir, and remdesivir (eg, NCT04336904, NCT04328285, and NCT04280705). Other trials are focusing on immunomodulators, including tocilizumab and anakinra (eg, NCT04317092 and NCT04330638).
WHO
Novel coronavirus (2019-nCoV) situation report 148.
,
Zimmermann P, Goetzinger F, Ritz N. Severe and fatal COVID-19 occurs in young children. JAMA Pediatrics (in press).
Most published data originate from China, which cannot necessarily be extrapolated to children in Europe and elsewhere.
Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding.
,
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team
The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.
,
- Chen H
- Guo J
- Wang C
- et al.
Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records.
,
- Zhu L
- Wang J
- Huang R
- et al.
Clinical characteristics of a case series of children with coronavirus disease 2019.
,
- Castagnoli R
- Votto M
- Licari A
- et al.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review.
,
SARS-CoV-2 infection in children.
,
Epidemiology of COVID-19 among children in China.
,
- Liu W
- Zhang Q
- Chen J
- et al.
Detection of Covid-19 in children in early January 2020 in Wuhan, China.
Also, existing papers from China contain very few clinical data on children, and most lack details regarding supportive measures required by children with COVID-19. Similarly, recent epidemiological reports from Europe and North America contain little clinically relevant information.
- Gudbjartsson DF
- Helgason A
- Jonsson H
- et al.
Spread of SARS-CoV-2 in the Icelandic population.
,
CDC COVID-19 Response Team
Coronavirus disease 2019 in children—United States, February 12–April 2, 2020.
Determining the level of support required by children is essential for paediatric service planning during the ongoing COVID-19 pandemic.
Evidence before this study
We searched MEDLINE on May 7, 2020, through the PubMed interface to identify publications describing clinical studies in children with COVID-19. To ensure a broad search, the search terms used were “(child OR children OR pediatric OR paediatric) AND COVID-19”. No additional limits were set. This search yielded 809 papers: 104 case reports or case series; 38 epidemiological reports; 66 guidelines and consensus statements; 184 reviews, perspectives, or editorials without original data; and 53 letters; 332 were unrelated to children with COVID-19. 22 papers presented original data, but exclusively in adults. Only ten papers reported clinical studies in children with COVID-19: eight papers originated from China, one from Spain, and one from Italy. The study by Tagarro and colleagues was reported in letter format, and only included 41 children with confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in Madrid. The study from Italy by Parri and colleagues was also reported as a letter and included 100 cases across several Italian hospitals. However, the study only featured a single patient who required mechanical ventilation, and consequently very few data on children with COVID-19 at the severe end of the disease spectrum.
Added value of this study
To our knowledge, this study is the first multinational, multicentre study in children with COVID-19, and provides a detailed overview on SARS-CoV-2 infection in children in Europe during the initial peak of the pandemic, which was facilitated by a collaboration of 82 units across 25 European countries. The study has several key findings. First, the data show that COVID-19 is generally a mild disease in children, including infants. Second, the study found that a substantial proportion (8%) of children develop severe disease, requiring intensive care support and prolonged ventilation. Several predisposing factors for requiring intensive care support were identified. Third, the study confirms that fatal outcome is rare in children. There was considerable variability in the use of drugs with antiviral activity as well as immunomodulatory medication, reflecting current uncertainties regarding specific treatment options.
Implications of all the available evidence
This study confirms previous reports from China suggesting that the case-fatality rate of COVID-19 in children is substantially lower than in older adult patients. However, some children develop severe disease and require prolonged intensive care support, which should be accounted for in the planning of health-care services and allocation of resources during the ongoing pandemic. Finally, the findings highlight that data on antiviral and immunomodulatory drugs are urgently needed from well designed, randomised controlled trials in children, to enable paediatricians to make evidence-based decisions regarding treatment choices for children with severe COVID-19.
By use of a well established research network, predominately comprising paediatric infectious diseases specialists and paediatric pulmonologists, the aim of this study was to rapidly capture key data on COVID-19 in children in Europe on a large scale, to aid physicians in Europe and in other geographical locations with service planning and allocation of resources.
Methods
Study design and participants
- Basu Roy R
- Thee S
- Blazquez-Gamero D
- et al.
Performance of immune-based and microbiological tests in children with TB meningitis in Europe—a multi-center Paediatric Tuberculosis Network European Trials Group (ptbnet) study.
,
- Noguera-Julian A
- Calzada-Hernandez J
- Brinkmann F
- et al.
Tuberculosis disease in children and adolescents on therapy with anti-tumor necrosis factor-alpha agents: a collaborative, multi-centre ptbnet study.
,
- Villanueva P
- Neth O
- Ritz N
- Tebruegge M
Paediatric Tuberculosis Network European Trials Group
Use of Xpert MTB/RIF Ultra assays among paediatric tuberculosis experts in Europe.
,
- Tebruegge M
- Ritz N
- Koetz K
- et al.
Availability and use of molecular microbiological and immunological tests for the diagnosis of tuberculosis in Europe.
,
- Tebruegge M
- Buonsenso D
- Brinkmann F
- et al.
European shortage of purified protein derivative and its impact on tuberculosis screening practices.
,
- Tebruegge M
- Bogyi M
- Soriano-Arandes A
- Kampmann B
Paediatric Tuberculosis Network European Trials Group
Shortage of purified protein derivative for tuberculosis testing.
—were invited to contribute cases of confirmed SARS-CoV-2 infection that had been managed at or managed remotely by their health-care institution (including individuals admitted to other hospitals or identified during community screening) before or during the study period. Any individual aged 18 years or younger with SARS-CoV-2 infection confirmed by RT-PCR was eligible for inclusion. A standardised data collection spreadsheet was used by collaborators to record data from their centre. All data were reviewed by three of the investigators (FG, BS-G, and MT), and any inconsistencies and other data queries were clarified with the reporting collaborators. Units that did not see any cases before or during the study period were asked to report the absence of cases fulfilling the inclusion criteria at the end of the study period. The study was done over a 3·5-week period, from April 1 to April 24, 2020.
The study was reviewed and approved by the ptbnet steering committee, and the human research ethics committees of the University of Bochum, Germany (19-6545-BR), the Hospital Gregorio Marañon, Spain (CEIM HGUGM-177/20), and the city of Vienna, Austria (EK 20–071-VK). The study was conducted in accordance with the Declaration of Helsinki and its subsequent amendments. No personal or identifiable data were collected during the conduct of this study.
Study definitions
A confirmed case was defined as a patient in whom SARS-CoV-2 was detected in any clinical sample (respiratory tract, blood, stool, or cerebrospinal fluid) by RT-PCR. PCR testing was done as part of routine clinical care, and therefore done according to local testing guidelines in place at the time. Date of symptom onset was defined as the day when the first symptom or sign occurred, and date of diagnosis as the day when SARS-CoV-2 was first detected. Pyrexia was defined as a body temperature at least 38·0°C. The index case was defined as the most likely source case based on history; if multiple family members were affected, the person who displayed symptoms first was recorded. Diagnosis of upper respiratory tract infection was based on clinical signs and symptoms, encompassing any of the following: coryza, pharyngitis, tonsillitis, otitis media, or sinusitis. Lower respiratory tract infection was based on clinical signs and auscultation findings. Inotropic support was defined as administration of dopamine, dobutamine, epinephrine, or norepinephrine by continuous infusion.
Statistical analysis
Non-parametric two-tailed Mann-Whitney U tests were used to compare continuous variables and χ2 or Fisher’s exact tests to compare categorical variables, as appropriate. In children younger than 2 years, age was calculated as fraction of a whole year (365 days); from 2 years of age, age was rounded to the nearest year. The 95% CI around the case-fatality rate (CFR) was calculated with the Wald method. Normality of data distribution was assessed with the Shapiro-Wilk test. The clinical endpoint was the need for admission to an intensive care unit (ICU; either neonatal or paediatric intensive care). The association of baseline characteristics and clinical findings with ICU admission was initially evaluated using univariable logistic regression. Subsequently, multivariable logistic regression analysis with the backward stepwise method was used to explore variables that were independently associated with ICU admission. Only variables that were significant in univariable analyses were introduced into the model. Factors associated with drug treatment for COVID-19 were also explored with univariable analysis. All probabilities are two tailed. p<0·05 was considered statistically significant. All analyses were done with Prism (version 8.0; GraphPad, La Jolla, CA, USA) and SPSS (version 25.0; IBM, Armonk, NY, USA).
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript. The corresponding author had full access to all the data and had the final responsibility for the decision to submit for publication.
Results
Figure 1Location of participating units and number of paediatric cases reported by country
82 participating units are shown; cities with more than one participating unit are represented by a single dot only (London [four units], Antwerp [n=3], Madrid [n=3], Vienna [n=3], Barcelona [n=2], Berlin [n=2], Girona [n=2], Manchester [n=2], Rome [n=2], Tallinn [n=2], and Zagreb [n=2]).
582 individuals with PCR-confirmed SARS-CoV-2 infection were included in the final analyses. 454 (78%) were contributed by tertiary or quaternary health-care institutions, whereas 54 (9%) had been diagnosed in secondary and 74 (13%) in primary health-care settings.
TableBaseline characteristics in the entire cohort and by requirement of ICU admission
Data are n (%), n/N (%), or median (IQR), unless stated otherwise. p values shown are based on univariable analyses, and calculated separately to the odds ratios. Odds ratios refer to the likelihood of admission to ICU, and were not calculated where one of the required values is zero. ICU=intensive care unit. ARDS=acute respiratory distress syndrome.
Figure 2Violin plots showing the age distribution of patients by requirement of ICU support
Each circle represents a patient. The solid lines represent the medians and dashed lines represent IQRs. ICU=intensive care unit.
437 (75%) individuals had no pre-existing medical conditions. Among the remaining 145 (25%) individuals, the most common conditions were chronic pulmonary disease (29 individuals, of whom 16 had asthma and six bronchopulmonary dysplasia), followed by malignancy (27 individuals, of whom 14 had leukaemia or lymphoma and 11 had solid tumours), neurological disorders (26 individuals, of whom nine had epilepsy and eight had cerebral palsy), congenital heart disease (25 individuals), chromosomal abnormalities (ten individuals, of whom eight had trisomy 21), and chronic kidney disease (nine individuals; table). 17 (3%) individuals had two or more pre-existing medical conditions.
29 (5%) individuals were receiving immunosuppressive medication at the time of COVID-19 diagnosis (table). Three (1%) had a previously diagnosed immunodeficiency, comprising common variable immunodeficiency, congenital neutropenia, and Schimke immuno-osseous dysplasia. 25 (4%) individuals were receiving chemotherapy at the time of their diagnosis or had received chemotherapy in the preceding 6 months. Three (1%) had previously undergone human stem cell transplant.
Pyrexia was the most common sign at presentation, observed in 379 (65%) individuals (table). Approximately half had signs or symptoms of upper respiratory tract infection and approximately a quarter had evidence of lower respiratory tract infection; 128 (22%) had gastrointestinal symptoms. 40 (7%) individuals with gastrointestinal symptoms had no respiratory symptoms; the majority (65%; n=26) of these individuals had pyrexia. 92 (16%) individuals were asymptomatic.
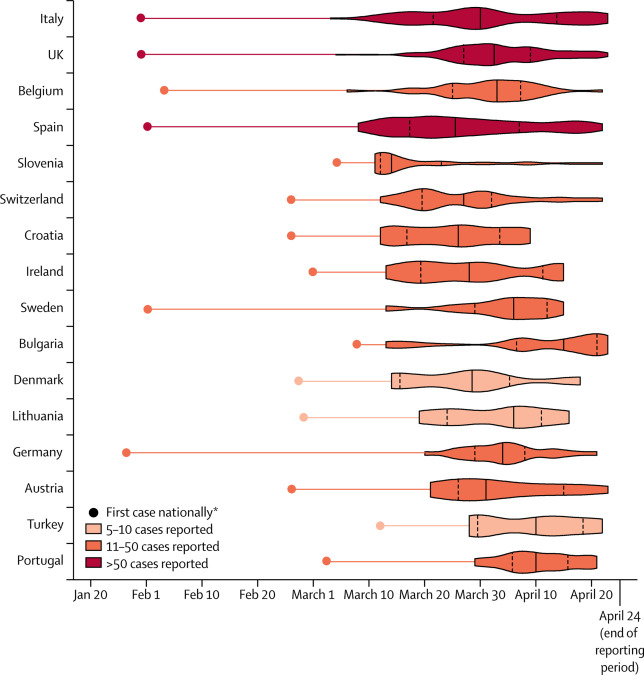
Figure 3Violin plot illustrating the dates SARS-CoV-2 infection was confirmed by RT-PCR in the study population, by country
Countries with fewer than five paediatric cases reported are not shown. Solid lines represent the medians and dashed lines represent IQRs. The date of the first case in each country is based on data reported by the European Centre for Disease Prevention and Control. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2. *First case of any age.
A chest x-ray was done in 198 (34%) patients. Of those, 93 (47%) had changes consistent with pneumonia (table). Ten (5%) had changes suggestive of acute respiratory distress syndrome (ARDS), all of whom required mechanical ventilation. In 29 (5%) patients, additional viruses were detected in respiratory samples, comprising enterovirus or rhinovirus (n=18), influenza virus (n=5), parainfluenza virus (n=3), adenovirus (n=3), respiratory syncytial virus (RSV; n=2), bocavirus (n=2), and coronavirus NL63, coronavirus HKU1, coronavirus OC43, and human metapneumovirus (n=1 each). In 22 patients one virus was detected in addition to SARS-CoV-2; in six patients, two additional viruses were detected simultaneously; and in one patient, three were detected. Patients with one or more viral co-infections were more likely to have signs or symptoms of upper or lower respiratory tract infection at presentation compared with those in whom no additional viral agent was identified (appendix p 1). Furthermore, individuals with viral co-infection were significantly more likely to require ICU admission, respiratory support, or inotropic support.
507 (87%) individuals did not require respiratory support at any stage. 75 (13%) patients required oxygen support: 31 (5%) were started on continuous positive airway pressure (CPAP) and 25 (4%) on mechanical ventilation (including 14 who had been managed with CPAP initially). The median duration of mechanical ventilation was 7 days (IQR 2–11; range 1–34). One (<1%) patient was started on extracorporeal membrane oxygenation. 19 (3%) patients required support with inotropes.
When comparing individuals by their requirement of ICU admission, we found that patients who required ICU admission were younger than those who did not (ie, individuals in the community and those admitted to hospital but not needing ICU support), but this was not statistically significant (table; figure 2). In univariable analysis, being younger than 1 month of age, male sex, pre-existing medical conditions, pyrexia, signs or symptoms of lower respiratory tract infection, radiological changes suggestive of pneumonia or ARDS, and viral co-infection were associated with ICU admission (table). In multivariable analysis, the factors that remained associated with ICU admission were being younger than 1 month (odds ratio [OR] 5·06, 95% CI 1·72–14·87; p=0·0035), male sex (2·12, 1·06–4·21; p=0·033), signs or symptoms of lower respiratory tract infection at presentation (10·46, 5·16–21·23; p
The most commonly used drug with antiviral activity was hydroxychloroquine, used in 40 (7%) patients, followed by remdesivir, which was used in 17 (3%) patients. Lopinavir–ritonavir was used in six (1%) patients and oseltamivir in three (1%), two of whom had influenza virus co-infection. Three (1%) patients received two drugs with antiviral activity and one (appendix p 2).
Four patients, all older than 10 years, had a fatal outcome (CFR 0·69%, 95% CI 0·20–1·82), with death occurring at 3, 9, 11, and 17 days after symptom onset. Two patients had no known pre-existing medical conditions; one had a cardiorespiratory arrest before arrival at the hospital and resuscitation was unsuccessful and the other died while being mechanically ventilated in ICU. The third patient had undergone human stem cell transplant 15 months earlier. The fourth patient was managed palliatively (without intubation), due to the severity of their pre-existing medical conditions. The remaining 578 patients were alive when the study closed. 93 (16%) individuals never developed clinical symptoms. In 460 (80%) individuals, all symptoms had resolved without apparent sequelae, whereas 25 (4%) were still symptomatic or were requiring respiratory support when the study closed.
Discussion
To our knowledge, this is the first multinational, multicentre study on paediatric COVID-19, and also the largest clinical study in children outside of China to date. The inclusion of such a substantial number of cases was made possible by involving a large number of specialist centres across Europe via a well established collaborative paediatric tuberculosis research network, allowing this study to provide one of the most detailed accounts of COVID-19 in children and adolescents published to date.
SARS-CoV-2 infection in children.
At the time our study was conducted, testing capacity for SARS-CoV-2 in many European countries was lower than clinical demand, and therefore many children with symptoms consistent with COVID-19 in the community were not tested and consequently not diagnosed. Nevertheless, our data indicate that children and adolescents are overall less severely affected by COVID-19 than adults, particularly older patients. Previous, large-scale data suggest that the CFR in adults older than 70 years is close to 10%,
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team
The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.
potentially due to immunosenescence.
- Malaguarnera L
- Ferlito L
- Imbesi RM
- et al.
Immunosenescence: a review.
It is reassuring that our data show that severe COVID-19 is uncommon in young children, including infants, despite their immune maturation being incomplete,
- Kollmann TR
- Kampmann B
- Mazmanian SK
- Marchant A
- Levy O
Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny.
,
- Kollmann TR
- Crabtree J
- Rein-Weston A
- et al.
Neonatal innate TLR-mediated responses are distinct from those of adults.
with only few requiring mechanical ventilation. It was striking that all children who died in our cohort were older than 10 years.
CDC COVID-19 Response Team
Coronavirus disease 2019 in children—United States, February 12–April 2, 2020.
The Australian Health Protection Agency has reported that children accounted for only 4% of confirmed COVID-19 cases in Australia.
COVID-19 National Incident Room Surveillance Team
COVID-19, Australia: epidemiology report 11 (reporting week 12 April 2020).
Unfortunately, in the CDC report, clinical data were only available in a small proportion of patients (n=291; 11%). In concordance with our observations, fever and cough were the predominant clinical features at presentation (present in 56% and 54% of individuals, respectively), with similar rates observed in a study from Italy.
- Parri N
- Lenge M
- Buonsenso D
Children with COVID-19 in pediatric emergency departments in Italy.
In our cohort almost a quarter of patients had gastrointestinal symptoms, some of whom had no respiratory symptoms, and a substantial proportion of children were entirely asymptomatic.
CDC COVID-19 Response Team
Coronavirus disease 2019 in children—United States, February 12–April 2, 2020.
but it is unclear how many patients were still hospitalised by the time of publication, so it is difficult to come to firm conclusions regarding the CFR in US children. Our data indicate that the CFR in children and adolescents across Europe is less than 1%. Considering that many children with mild disease will never have been brought to medical attention, and therefore not diagnosed, it is highly probable that the true CFR is substantially lower than the figure of 0·69% observed in our cohort. This hypothesis is further supported by an epidemiological study from China, in which the CFR in individuals aged 19 years or younger was only 0·1% (one death in 965 confirmed cases).
Novel Coronavirus Pneumonia Emergency Response Epidemiology Team
The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.
Furthermore, our data indicate that sequelae related to COVID-19 are likely to be rare in children and adolescents. However, after the closure of our study, reports of a hyperinflammatory syndrome affecting children that is temporally, and potentially causally, associated with SARS-CoV-2 infection have emerged, which has subsequently been named paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS; sometimes known as MIC-S).
- Riphagen S
- Gomez X
- Gonzalez-Martinez C
- Wilkinson N
- Theocharis P
Hyperinflammatory shock in children during COVID-19 pandemic.
,
European Centre for Disease Prevention and Control
Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children.
Further research will be required to characterise this emerging disease entity in detail, and determine the long-term outcome of affected children.
- Tagarro A
- Epalza C
- Santos M
- et al.
Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain.
In our cohort, being younger than 1 month, male sex, presence of lower respiratory tract infection signs or symptoms at presentation, and presence of a pre-existing medical condition were associated with increased likelihood of requiring ICU admission. Our results also show that the majority of children who are intubated due to respiratory failure require prolonged ventilation, often for 1 week or more. This contrasts with observations in children with RSV infection who, on average, only require mechanical ventilation for 5–7 days,
- McKiernan C
- Chua LC
- Visintainer PF
- Allen H
High flow nasal cannulae therapy in infants with bronchiolitis.
but is not dissimilar to observations in children with influenza.
- von der Beck D
- Seeger W
- Herold S
- Gunther A
- Loh B
Characteristics and outcomes of a cohort hospitalized for pandemic and seasonal influenza in Germany based on nationwide inpatient data.
This has important implications for service planning, as although the overall demand for ICU support might be lower in children than in adults, each patient is likely to occupy ICU space for an extended period of time. At this time of intense strain on health-care services worldwide, it is vital that adequate resources are allocated to paediatric services to sustain the provision of high-quality care for children.
The observation that, in our study, individuals with viral co-infection (ie, infected with SARS-CoV-2 and one or more other viral agents) were more likely to require ICU support than those in whom SARS-CoV-2 was the only viral agent identified might have implications for the winter period 2020–21, when the incidence of other viral respiratory tract infections, including RSV and influenza virus infections, is bound to increase. This could result in a greater proportion of paediatric patients with COVID-19 requiring ICU support than in the cohort described here, as the influenza season 2019–20 was already over in Europe before the study commenced.
- Chiotos K
- Hayes M
- Kimberlin DW
- et al.
Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2.
Overall, the expert panel appeared to favour the use of remdesivir over other agents, based on the currently available data from in-vitro and animal studies, including in non-human primates, and recent data from compassionate use in humans.
- Grein J
- Ohmagari N
- Shin D
- et al.
Compassionate use of remdesivir for patients with severe COVID-19.
,
Therapeutic options for the 2019 novel coronavirus (2019-nCoV).
The panel members’ opinion was split regarding the use of lopinavir–ritonavir, given the disappointing results of a recently published randomised controlled trial.
- Cao B
- Wang Y
- Wen D
- et al.
A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19.
The main limitation of this study relates to the number of variables for which data were collected. In the context of the ongoing COVID-19 pandemic, to ensure high levels of participation and avoid diverting substantial time away from clinical front-line duties, a decision was made not to collect detailed data on laboratory parameters or ICU interventions. A further limitation was that a variety of in-house and commercial PCR assays were used across different participating centres, precluding an assessment of diagnostic test performance. Also, the number of children receiving antiviral or immunomodulatory treatment was too small to draw meaningful conclusions regarding their effectiveness, which will be addressed by the aforementioned randomised trials. A further limitation is that different countries were using different thresholds to screen for SARS-CoV-2 at the time the study was done, with some recommending screening of all children admitted to hospital or conducting community screening, whereas others were using more selective testing strategies. Despite those limitations, to our knowledge, this study provides the most comprehensive overview on COVID-19 in children and adolescents to date.
In conclusion, our data, originating from a large number of specialist centres across Europe, show that COVID-19 is usually a mild disease in children, including infants. Nevertheless, a small proportion of children and adolescents develop severe disease and require ICU support, frequently needing prolonged ventilatory support. However, fatal outcome is rare overall. Our data also reflect the current uncertainties regarding specific treatment options, highlighting that more robust data on antiviral and immunomodulatory drugs are urgently needed.
Contributors
MT conceived of the study. FG, BS-G, SBW, MB, FB, and MT designed the study. FG, BS-G, and MT cleaned and analysed the data, constructed the figures, and wrote the first draft of the manuscript. All authors contributed data to the study, contributed to the data interpretation, critically reviewed the manuscript, and approved the final manuscript for submission.
ptbnet COVID-19 Study Group
Jasmin Pfefferle and Angela Zacharasiewicz (Wilhelminenspital, Vienna, Austria), Angelika Berger (Medical University Vienna, Vienna, Austria), Roland Berger (St Josef Hospital, Vienna, Austria), Volker Strenger and Daniela S Kohlfürst (Department of Paediatrics and Adolescent Medicine, Medical University of Graz, Graz, Austria), Anna Zschocke and Benoît Bernar (Department of Pediatrics, Medical University Innsbruck, Innsbruck, Austria), Burkhard Simma (Landeskrankenhaus Feldkirch, Feldkirch, Austria), Edda Haberlandt (Krankenhaus Dornbirn, Dornbirn, Austria), Christina Thir and Ariane Biebl (Kepler University Hospital, Linz, Austria), Koen Vanden Driessche and Tine Boiy (Antwerp University Hospital, Antwerp, Belgium), Daan Van Brusselen (GZA Hospitals Antwerp, Antwerp, Belgium), An Bael (ZNA Paola Children’s Hospital Antwerp & University of Antwerp, Antwerp, Belgium), Sara Debulpaep and Petra Schelstraete (Gent University Hospital, Gent, Belgium), Ivan Pavić (Children’s Hospital Zagreb, Zagreb, Croatia), Ulrikka Nygaard (Copenhagen University Hospital, Denmark), Jonathan Peter Glenthoej (Nordsjaellands Hospital, Hilleroed, Denmark), Lise Heilmann Jensen (Zealand University Hospital, Roskilde, Denmark), Ilona Lind (Pärnu Hospital, Pärnu, Estonia), Mihhail Tistsenko (West-Tallinn Central Hospital, Tallinn, Estonia), Ülle Uustalu (Tallinn Children’s Hospital, Tallinn, Estonia), Laura Buchtala (Klinikum Bremen-Mitte, Bremen, Germany), Stephanie Thee (Charité Universitätsmedizin, Berlin, Germany), Robin Kobbe and Cornelius Rau (University Children’s Hospital, University Medical Center Hamburg-Eppendorf, Hamburg, Germany), Nicolaus Schwerk (Hannover Medical School, Hannover, Germany), Michael Barker (Helios Klinikum Emil von Behring, Berlin, Germany), Maria Tsolia and Irini Eleftheriou (2nd Department of Paediatrics, National & Kapodistrian University of Athens, P & A Kyriakou Children’s Hospital, Greece), Patrick Gavin and Oksana Kozdoba (Children’s Health Ireland at Crumlin and Temple Street, Dublin, Ireland), Borbàla Zsigmond (Heim Pal Children’s Hospital, Budapest, Hungary), Piero Valentini (Fondazione Policlinico Universitario A Gemelli IRCCS, Rome, Italy), Inga Ivaškevičienė and Rimvydas Ivaškevičius (Clinic of Children’s Diseases, Institute of Clinical Medicine, Vilnius University, Vilnius, Lithuania), Valentina Vilc (Institute of Phthisiopneumology, Chisinau, Moldova), Elisabeth Schölvinck (Beatrix Children’s Hospital University Medical Centre Groningen, Groningen, The Netherlands), Astrid Rojahn (Oslo University Hospital, Oslo, Norway), Anastasios Smyrnaios (St Olavs University Hospital, Trondheim, Norway), Claus Klingenberg (University Hospital of North Norway, Tromso, Norway), Isabel Carvalho and Andreia Ribeiro (Hospital Center of Vila Nova de Gaia/Espinho, Porto, Portugal), Anna Starshinova (Almazov National Medical Research Centre, St Petersburg, Russia), Ivan Solovic (National Institute for Tuberculosis, Lung Diseases and Thoracic Surgery, Vysne Hagy, Slovakia), Lola Falcón and Olaf Neth (Hospital Infantil Virgen del Rocío, Sevilla, Spain), Mario Pérez-Butragueño (Hospital Universitario Infanta Leonor, Madrid, Spain), Laura Minguell (Hospital Universitari Arnau de Vilanova, Lleida, Spain), Matilde Bustillo and Aida María Gutiérrez-Sánchez (Miguel Servet University Hospital, Zaragoza, Spain), Borja Guarch Ibáñez (Hospital Universitari de Girona Dr Josep Trueta, Girona, Spain), Francesc Ripoll (Hospital Santa Caterina, Girona, Spain), Beatriz Soto (Hospital Universitario de Getafe, Madrid, Spain), Karsten Kötz (Queen Silvia Children’s Hospital, Gothenburg, Sweden), Petra Zimmermann (Hôpital Fribourgeois, Fribourg, Switzerland), Hanna Schmid (University Children’s Hospital Basel, Basel, Switzerland), Franziska Zucol (Kantonsspital Winterthur, Winterthur, Switzerland), Anita Niederer (Children’s Hospital of Eastern Switzerland, St Gallen, Switzerland), Michael Buettcher (Lucerne Children’s Hospital, Lucerne Cantonal Hospital, Lucerne, Switzerland), Benhur Sirvan Cetin (Erciyes University Hospital, Kayseri, Turkey), Olga Bilogortseva (National Institute of Phthisiology and Pulmonology, Kiev, Ukraine), Vera Chechenyeva (National Specialised Children’s Centre for HIV & AIDS, Kiev, Ukraine), Alicia Demirjian (Evelina London Children’s Hospital, Guy’s and St Thomas’ NHS Foundation Trust, London, UK), Fiona Shackley (Sheffield Children’s Hospital, UK), Lynne McFetridge (Antrim Area Hospital, Antrim, UK), Lynne Speirs (Royal Belfast Hospital for Sick Children, Belfast, UK), Conor Doherty (Royal Hospital for Children, Glasgow, UK), Laura Jones (Royal Hospital for Sick Children, Edinburgh, UK), Paddy McMaster (North Manchester Care Organisation, Manchester, UK), Clare Murray and Frances Child (Royal Manchester Children’s Hospital, Manchester University NHS Foundation Trust, Manchester, UK), Yvonne Beuvink and Nick Makwana (City and Sandwell Hospitals Birmingham, Birmingham, UK), Elisabeth Whittaker (St Mary’s Hospital Paddington, London, UK), Amanda Williams (London North West University Healthcare NHS Trust, Harrow, UK), Katy Fidler (Royal Alexandra Children’s Hospital, Brighton, UK), Jolanta Bernatoniene (Bristol Royal Hospital for Children, Bristol, UK), Rinn Song and Zoe Oliver (Oxford Children’s Hospital, Oxford, UK), Andrew Riordan (Alder Hey Children’s Hospital, Liverpool, UK).
Declaration of interests
FG has received funding from Gilead for research related to hepatitis E. BS-G and MT have received assays free of charge from Cepheid for tuberculosis diagnostics projects. MT has received assays at reduced pricing or free of charge from Cellestis/Qiagen for tuberculosis diagnostics projects, has received support for conference attendance from Cepheid, and is currently receiving funding from bioMérieux as an investigator of an ongoing tuberculosis diagnostics study. UH reports personal fees from CEPI for being a member of the SPEAC-CEPI Meta-Data safety monitoring board for COVID-19 vaccine trials, outside of the submitted work. The other authors declare no competing interests.
Acknowledgments
We express our gratitude to all colleagues and research personnel involved in the data collection for this study, as well as the members of the human research ethics committees and institutional review boards that have kindly fast-tracked this study. We are also grateful for the kind support of the Clinical Microbiology & Infectious Diseases Department and the COVID-19 Group at Hospital General Universitario Gregorio Marañón, Madrid, Spain. This project did not receive specific funding. ptbnet is supported by the Deutsche Gesellschaft für Internationale Zusammenarbeit. BS-G is funded by the Spanish Ministry of Health—Instituto de Salud Carlos III and co-funded by the European Union (FEDER; Contrato Juan Rodés, Grant JR16/00036). AN-J was supported by “Subvencions per a la Intensificacio de Facultatius Especialistes”—Departament de Salut de la Generalitat de Catalunya, Programa PERIS 2016–2020 (SLT008/18/00193).
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Supplementary Material
References
- 1.
Novel coronavirus (2019-nCoV) situation report 5.
- 2.
The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2.
Nat Microbiol. 2020; 5: 536-544
- 3.
Novel coronavirus (2019-nCoV) situation report 148.
- 4.
Zimmermann P, Goetzinger F, Ritz N. Severe and fatal COVID-19 occurs in young children. JAMA Pediatrics (in press).
- 5.
Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding.
Nat Med. 2020; 26: 502-505
- 6.
The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China.
Zhonghua Liu Xing Bing Xue Za Zhi. 2020; 41: 145-151
- 7.
Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records.
Lancet. 2020; 395: 809-815
- 8.
Clinical characteristics of a case series of children with coronavirus disease 2019.
Pediatr Pulmonol. 2020; 55: 1430-1432
- 9.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in children and adolescents: a systematic review.
JAMA Pediatr. 2020; ()
- 10.
SARS-CoV-2 infection in children.
N Engl J Med. 2020; 382: 1663-1665
- 11.
Epidemiology of COVID-19 among children in China.
Pediatrics. 2020; 145e20200702
- 12.
Detection of Covid-19 in children in early January 2020 in Wuhan, China.
N Engl J Med. 2020; 382: 1370-1371
- 13.
Spread of SARS-CoV-2 in the Icelandic population.
N Engl J Med. 2020; ()
- 14.
Coronavirus disease 2019 in children—United States, February 12–April 2, 2020.
MMWR Morb Mortal Wkly Rep. 2020; 69: 422-426
- 15.
Performance of immune-based and microbiological tests in children with TB meningitis in Europe—a multi-center Paediatric Tuberculosis Network European Trials Group (ptbnet) study.
Eur Respir J. 2020; ()
- 16.
Tuberculosis disease in children and adolescents on therapy with anti-tumor necrosis factor-alpha agents: a collaborative, multi-centre ptbnet study.
Clin Infect Dis. 2019; ()
- 17.
Use of Xpert MTB/RIF Ultra assays among paediatric tuberculosis experts in Europe.
Eur Respir J. 2018; 51800346
- 18.
Availability and use of molecular microbiological and immunological tests for the diagnosis of tuberculosis in Europe.
PLoS One. 2014; 9e99129
- 19.
European shortage of purified protein derivative and its impact on tuberculosis screening practices.
Int J Tuberc Lung Dis. 2016; 20: 1293-1299
- 20.
Shortage of purified protein derivative for tuberculosis testing.
Lancet. 2014; 3842026
- 21.
Immunosenescence: a review.
Arch Gerontol Geriatr. 2001; 32: 1-14
- 22.
Protecting the newborn and young infant from infectious diseases: lessons from immune ontogeny.
Immunity. 2017; 46: 350-363
- 23.
Neonatal innate TLR-mediated responses are distinct from those of adults.
J Immunol. 2009; 183: 7150-7160
- 24.
COVID-19, Australia: epidemiology report 11 (reporting week 12 April 2020).
Commun Dis Intell. 2020; 44
- 25.
Children with COVID-19 in pediatric emergency departments in Italy.
N Engl J Med. 2020; ()
- 26.
Hyperinflammatory shock in children during COVID-19 pandemic.
Lancet. 2020; 395: 1607-1608
- 27.
Rapid risk assessment: paediatric inflammatory multisystem syndrome and SARS-CoV-2 infection in children.
- 28.
Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain.
JAMA Pediatr. 2020; ()
- 29.
High flow nasal cannulae therapy in infants with bronchiolitis.
J Pediatr. 2010; 156: 634-638
- 30.
Characteristics and outcomes of a cohort hospitalized for pandemic and seasonal influenza in Germany based on nationwide inpatient data.
PLoS One. 2017; 12e0180920
- 31.
Multicenter initial guidance on use of antivirals for children with COVID-19/SARS-CoV-2.
J Ped Infect Dis Soc. 2020; ()
- 32.
Compassionate use of remdesivir for patients with severe COVID-19.
N Engl J Med. 2020; ()
- 33.
Therapeutic options for the 2019 novel coronavirus (2019-nCoV).
Nat Rev Drug Discov. 2020; 19: 149-150
- 34.
A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19.
N Engl J Med. 2020; 382: 1787-1799
Article Info
Publication History
Identification
DOI: https://doi.org/10.1016/S2352-4642(20)30177-2
Copyright
© 2020 Elsevier Ltd. All rights reserved.
ScienceDirect
Access this article on ScienceDirect
